Let us assume that a system is changing with respect to pressure as well as volume. Let the ‘X’ be the initial state of the system and ‘Y’ is the final state of the system. Now let suppose UX and UY are the energies of the system in its state ‘X’ and ‘Y’ respectively. Hence Change in internal energy will be given by:
ΔU = UY – UX
Now let us assume that system changes from state X to state Y by following Path I and change in energy is accompanied by ΔU.
Now suppose that by using another path II the same change of state is brought and energy change associated with this be ΔU’.
Let, ΔU is greater than ΔU’ i.e.
ΔU> ΔU’
By coupling of these two processes:
X ——-> Y (by path I) and
Y ——-> X (by path II)
It is shown in figure below
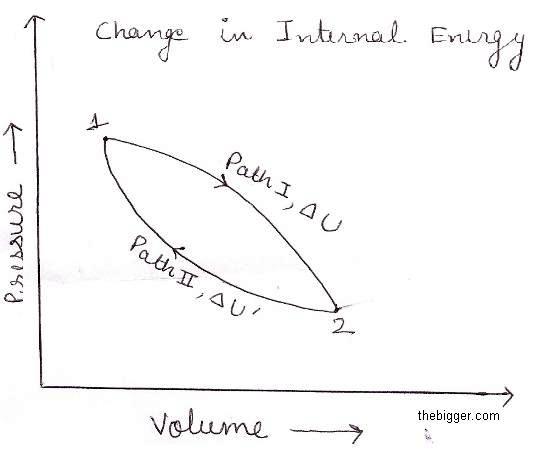
The system will come back to its initial state and at the same time the energy which is used will b available which is equal to ΔU- ΔU’. Repeating the same cycle again and again , continuous energy will be generated and perpetual motion will become possible. This is contradiction to the first law which states that energy can neither be created nor be destroyed.
Hence,
ΔU = ΔU’
Hence change of energy is accompanied as a function of initial and final states of the system only and it is independent of the path i.e. it does not matter how the change is brought about.
