The state at which the concentrations of reactants and products do not change with of a chemical reaction is known as chemical equilibrium. When the reactants are mixed together in a vessel, the whole reactants do not completely converted into products. After some time the rate of formation products from reactants and rate of formation of reactants from products becomes equal and that state is known as chemical equilibrium.
The most important aspect of equilibrium system is the reversibility. In equilibrium, both the forward and the backward reactions are still taking place. But the rate of forward and the backward reactions become equal. Hence, the concentrations of each species become constant.
For example:
Equilibrium in decomposition of calcium carbonate, reaction between hydrogen and iodine, etc
Decomposition of calcium carbonate:
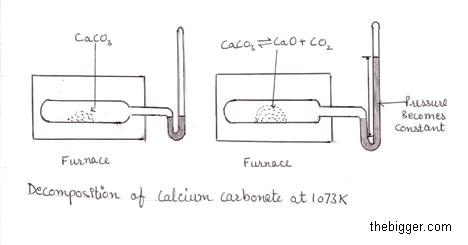
When we heat solid calcium carbonate in a closed vessel at a temperature of 1073 K, it decomposes into calcium oxide(solid) and CO2(gaseous ). CO2 produced in the above reaction will exert some pressure in the vessel. This pressure is measured with the help of a manometer as shown below. The reading on manometer shows that as the process of decomposition continues the pressure value increases but till a certain limit. Once that limit is reached, pressure becomes constant at a given constant temperature. This means that CO2 production has become constant despite some CaCO3 presence. This stabilizing of the pressure (CO2 production becoming constant) signifies that we have reached the equilibrium.
This can be represented as:
CaCO3 ⇌ CaO (s) + CO2 (g)