We know that for an ideal gas, work done w is given as:
Wideal = -nRT ln (V2/ V1)
And for a a van der Waals Gas, work done is given as:
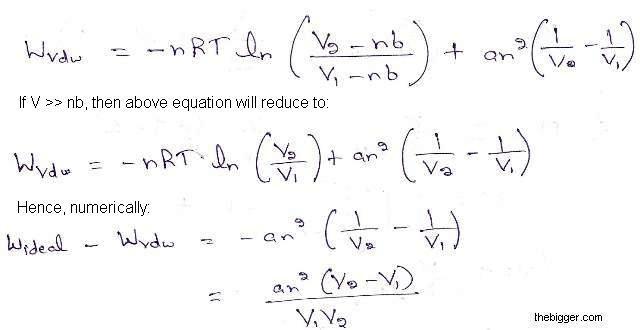
Hence for the expansion of a gas, V2 > V1, which shows that numerically the work of expansion in the reversible isothermal expansion of an ideal gas is greater than van der Waals Gas.


