The term conservation of electric charges means that charges can neither be created nor destroyed in separation. In simple words, charges can be created or destroyed only in same and opposite pairs.
When two bodies are rubbed together then one gets negative and other gets positive charge. It is clear from the fact that charges are not produced due to rubbing of bodies; charges are transferred from one body to another by disturbing the electrical neutrality of the bodies.
Examples that show the conservation of charge are:
1. In all the processes charge number is same every time.
2. In the circumstances of pair generation, a gamma photon divides into an electron and a positron. Then also the total charge –e+e=0. That is the total charge present on a photon.
Difference between charge and mass.
|
Charge |
Mass |
| Velocity does not affect the charge on a body
Charge can be negative or positive. Charges are quantized. Electric charge is always conserved In case of charge, both attractive as well as repulsive forces work. |
Mass of a body increases with velocity.
Mass is always positive quantity. Quantization of mass has not been founded yet. Mass can not be changed into energy and other quantities, so conservation of mass is not possible. In some cases of mass, gravitational force works which is always attractive. |
Coulombs’ Law
It states that, the force of interaction between two point charges is inversely proportional to the square of distance between charges and directly proportional to the product of charges.
Suppose q1 and q2 are the charges on two bodies, set apart in vacuum from each other by a distance r, and the dimensions of both the bodies are smaller that the distance (r) between them. So, we can consider them as point charges. According to Coulomb’s law,
Or
In the above figure, k is the electrostatic force constant.
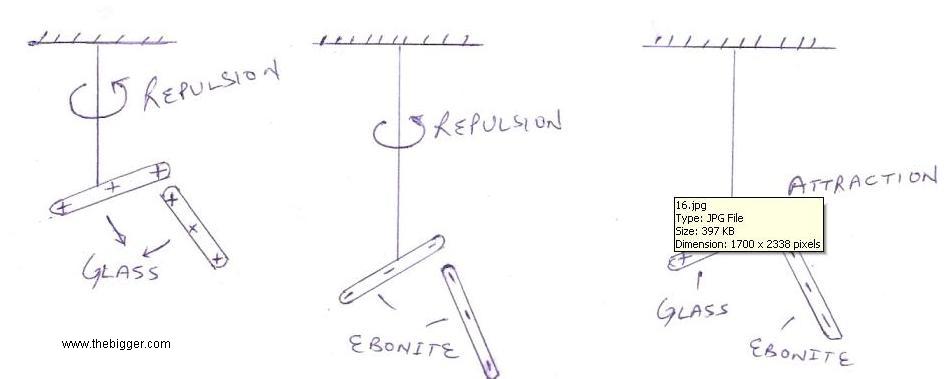
If q1 q2 > 0 then it means that both the charges on the bodies are same i.e. both negative or both positive. Then the bodies will repel each other.
The force
represents the force applied on charge q1 by q2.
Acc to coulombs law…
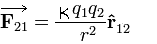 ——— 1.1
——— 1.1
Where k is electrostatic force constant.
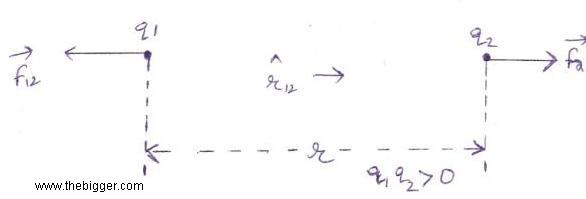
Similarly the force applied on charge q1 by q2 is shown below:
is the unit vector indicating from q2 to q1.
If one of the charge is negative and the other charge is positive i.e. q1 q2 <0, then both the bodies will attract each other.
These forces can also be written again as :
And
As
Therefore
It is clear from the above equation that the force applied by the charges on one another is always opposite and equal. Therefore, the above result also follows the Newton’s Third law of motion. So, the Coloumbs forces of interaction are always equidistant to the line connecting the centres of the two charged bodies.So, electrostatic forces are also said to be conservative forces.
Note: Coloumbs law is only applicable to point charges.
The value of the electrostatic constant k varies with the change in the separation medium between the two charges.
In vaccum, the value of k calculated according to the c.g.s. system is 1.
In S.I. units we write k as
Where
is absolute electrical permittivity of the vaccum.
Experimental value of k is :
9 X 109 Nm2C-2
Therefore
= 8.85 X 10-12C2N-1m-2
From the above figure 1.1 coulombs law in SI units is:
———- 1.2
So the magnitude of the above equation is:
———–1.3
Things To Remember
• Coloumbs law is applicable to the charges in which the distances extends from nuclear dimensions (approx. 10 -15 m) to several kilometres. So, we can say that if in Coulombs law, the distance between two bodies r is less than 10-15m ,then Coloumbs law will not remain valid.
• Coulombs law of electrostatic forces is almost similar to the Newton’s law of gravitational forces between the two masses.
i.e.
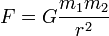
• Electrostatic forces between the charges are much more stronger than the gravitational forces, e.g. A charged glass rod after rubbing it with some silk cloth or a piece of fur has the ability to attract small pieces of paper, dust particles etc against the gravitational pull of earth. According to the theory of ‘Feyman’, if you are standing at some distance i.e. about an arm’s length from your friend. In place of being neutral if you had large no. of electrons over protons near about a single percent. Then the force of repulsion between you and your friend will be enough to lift the whole earth.
|
Charge |
Mass |
|
|


