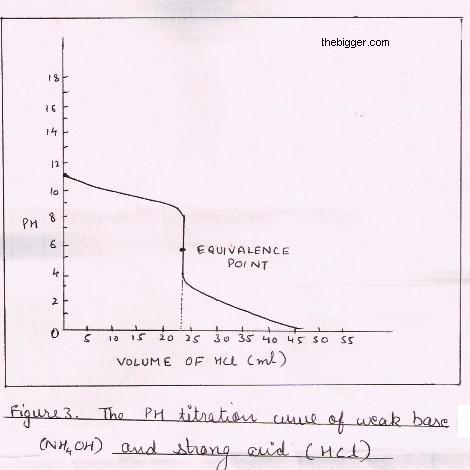
Titration of Strong Acid and a Weak Base:
For explaining this type of titration let us consider an example of HCl which is strong acid and ammonium hydroxide i.e. NH4OH which is a weak base. The reaction can be shown as:
NH4OH (aq) + HCl (aq) ———> NH4Cl (aq) + H2O (l)
In this case the curve is similar to that in weak acid and strong base as shown in figure below. However, the equivalence point is below 7 due to the reaction of NH4Cl formed at the neutralization with water. This reaction gives H+ ions. Also the equivalence point lies at pH=5.3 which makes it important to use an indicator which has an acidic ph for example:- Methyl orange. Though phenolphthalein suits the ph criteria but still is avoided as its color changes away from the equivalence point.